
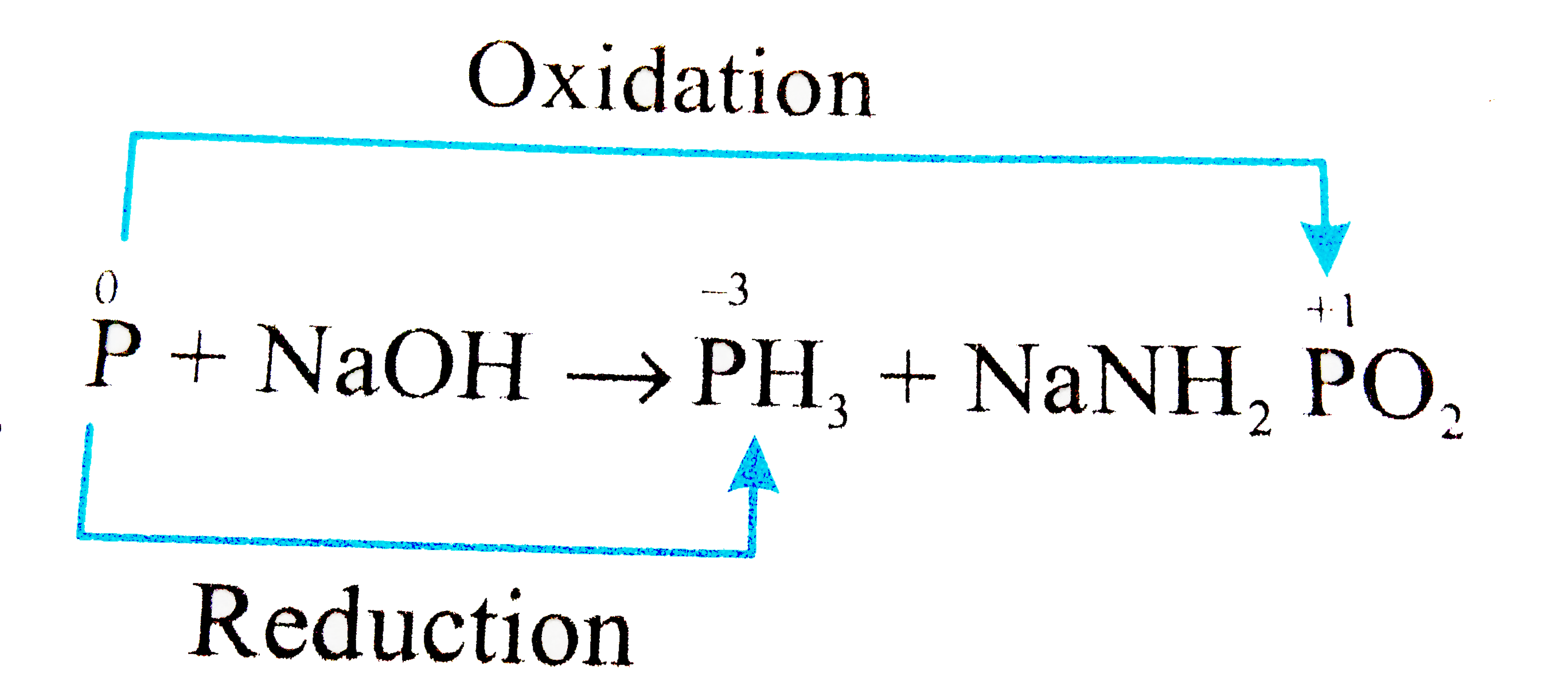
The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type, the oxidation states of phosphorus in phosphine and the other product are respectively:

i) Out of and which one is more reactive towards S N 1 and why? (ii) Write the product formed when p-nitrochlorobenzene is heated with aqueos NaOH at 443 K followed by

Water | Free Full-Text | Phosphorus Forms and Associated Properties along an Urban–Rural Gradient in Southern China
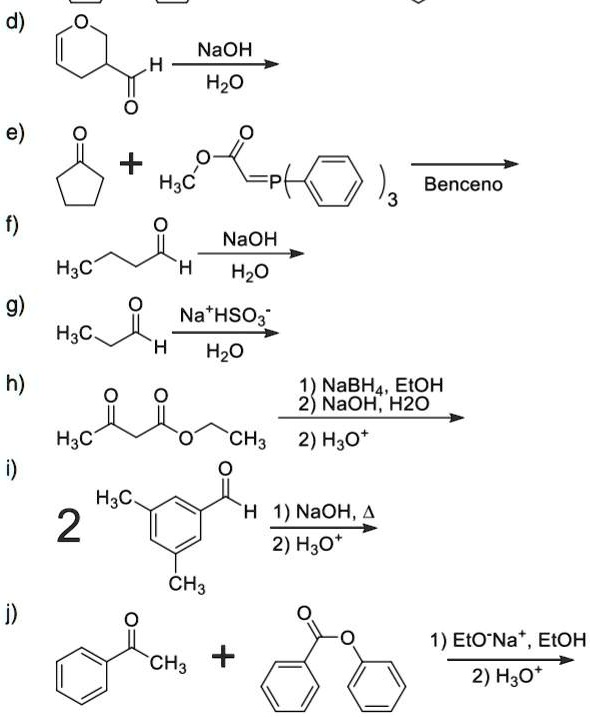
SOLVED: (p NaOH HzO e) H3l 13 Benceno f) NaOH HzO H3C (6 HaC NatHSO3 HzO h) NaBH4, EtOH NaOH; H2O CH3 2) Hjot H3C i) H3C N 1) NaOH; 2) Hzot
How should I balance this equation P + NaOH + H2O---------> PH3 + NaH2PO2 by ion electron method? - Quora

Unexpected Complexity in the Products Arising from NaOH-, Heat-, Amine-, and Glycosylase-Induced Strand Cleavage at an Abasic Site in DNA | Chemical Research in Toxicology

The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound. The reaction type; the oxidation states of phosphorus in phosphine and the other product are respectively.

Write an equation for the reaction of p-bromobenzaldeyde with HCN (NaOH as the catalyst). | Homework.Study.com

White phosphorus on reaction with concentrated NaOH solution in an inert atmosphere of CO2 gives phosphine and compound (X) . (X) on acidification with HCl gives compound (Y) . The basicity of

P and Q are aqueous solutions of sodium chloride and sodium hydroxide, respectivley. Which of these - YouTube

Write the product formed when p-nitrochlorobenzene is heated with aqueous naoh at 443k followed by - Brainly.in

Balance the given redox reaction using oxidation method :- P4 + NaOH -- PH3 + NaH2PO2 + H2O Pls - Chemistry - Redox Reactions - 14050673 | Meritnation.com

Effects of sodium hydroxide's concentration and time on the yields of... | Download Scientific Diagram

Write the Product Formed When P-nitro Chlorobenzene is Heated with Aqueous Naoh at 443k Followed by Acidification? - Chemistry | Shaalaa.com

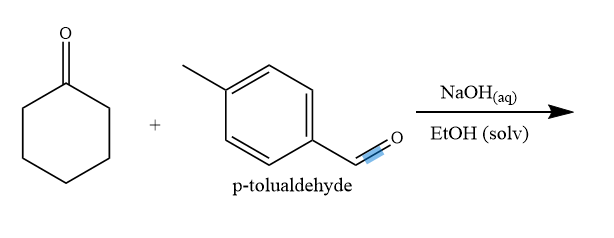
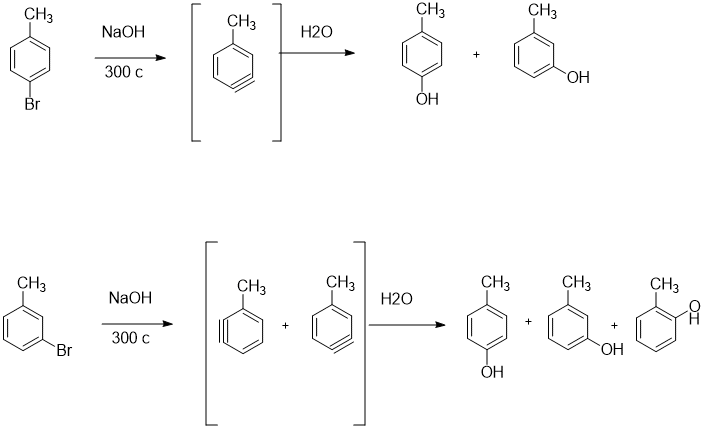

![ANSWERED] Consider the following three solutions of... - Physical Chemistry ANSWERED] Consider the following three solutions of... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59737718-1659709481.9039986.jpeg)